
CARESTO® heal
Stent
Braided carotid stent
with HEAL Technology

CARESTO® heal
Stent
Braided, self-expanding nitinol stent with HEAL Technology
- First coated carotid stent - with unique HEAL Technology
- Highly flexible, single layer closed-cell stent
- Small pore size with plaque coverage
- Flexible low profile delivery system (0.068" OD)
- Excellent visibility due to nitinol composite wires with platinum core
- Available in cylindrical and tapered version
- CE mark approved for vessel diameters from 4.0 mm – 10.0 mm
Note:
The CARESTO® heal Stent is currently in Limited Market Release. Please contact your Acandis® representative for product availability.
Note: Magnetic Resonance Imaging (MRI) Information
Non-clinical tests have shown that the Acandis implant is suitable for MR-examination. After implantation, patients can be safely scanned with a static magnetic field of 3 Tesla. The MR imaging quality may be affected if the implant is located in the area of interest. Optimisation of the imaging parameters is recommended. For further information on MRI compatibility please consult the instructions for use of the respective product.
Clinical Results
Find publications and clinical studies on our products.
Clinical Experience
with CARESTO® heal Stent
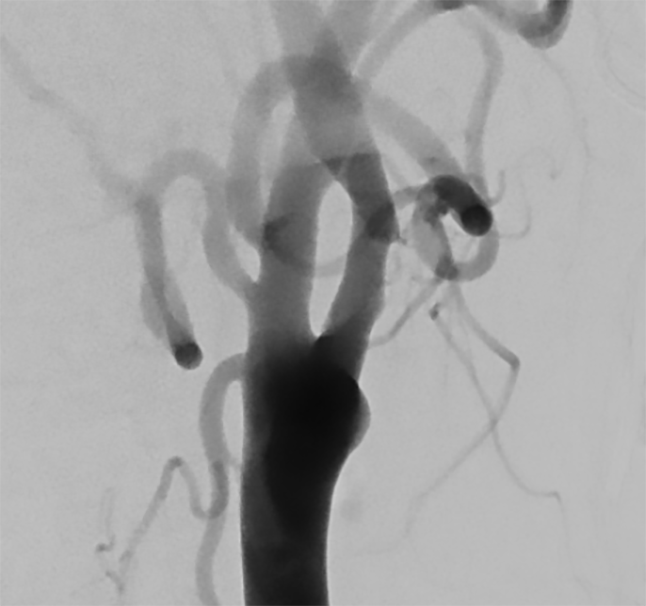
DSA prior to stenting confirms the rugged, middle stenosed primal ICA
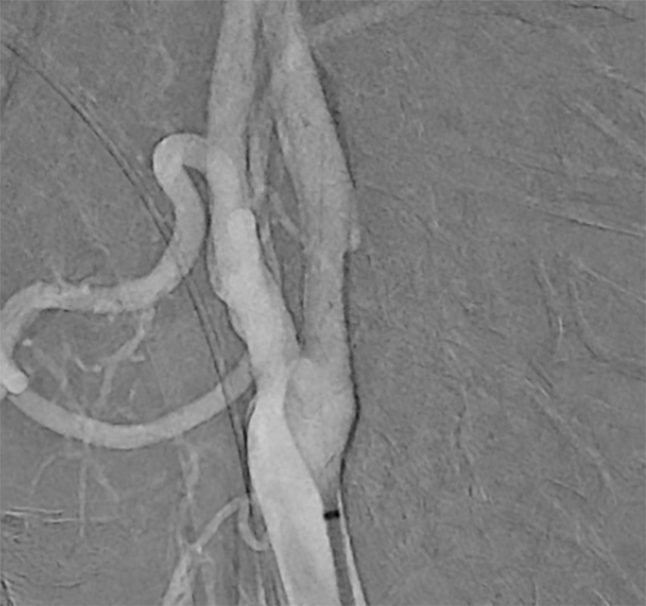
Deployment of CARESTO® heal Stent over
ICA stenosis
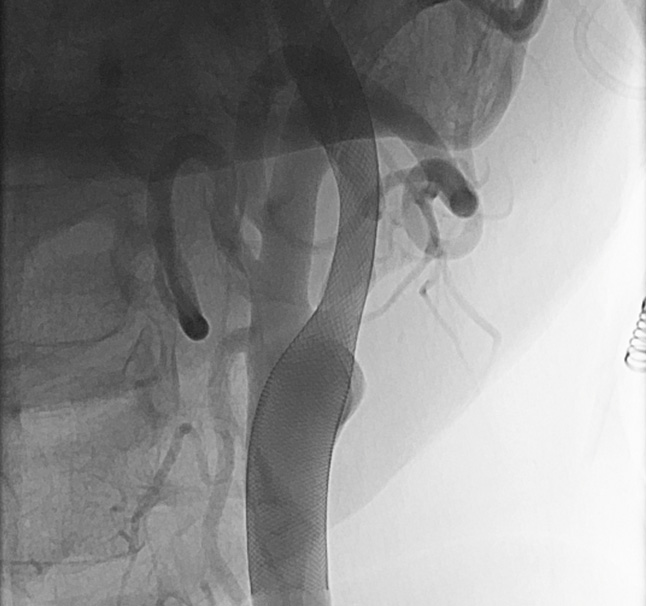
Control of placed CARESTO® heal Stent
Images are courtesy of Dr. Hannes Nordmeyer, radprax MVZ Solingen, Germany
Contact
Clinical Support (clinical-support@acandis.com)
Disclaimer:
Please consult the Instructions for Use for all indications, contraindications, warnings, cautions as well as possible adverse effects. Acandis® products are to be used exclusively by trained medical professionals. Orders are taken only in regions where the product is approved. Please contact an Acandis® representative for product availability.