
ACCLINO® flex plus
Stent
for flexible aneurysm treatment
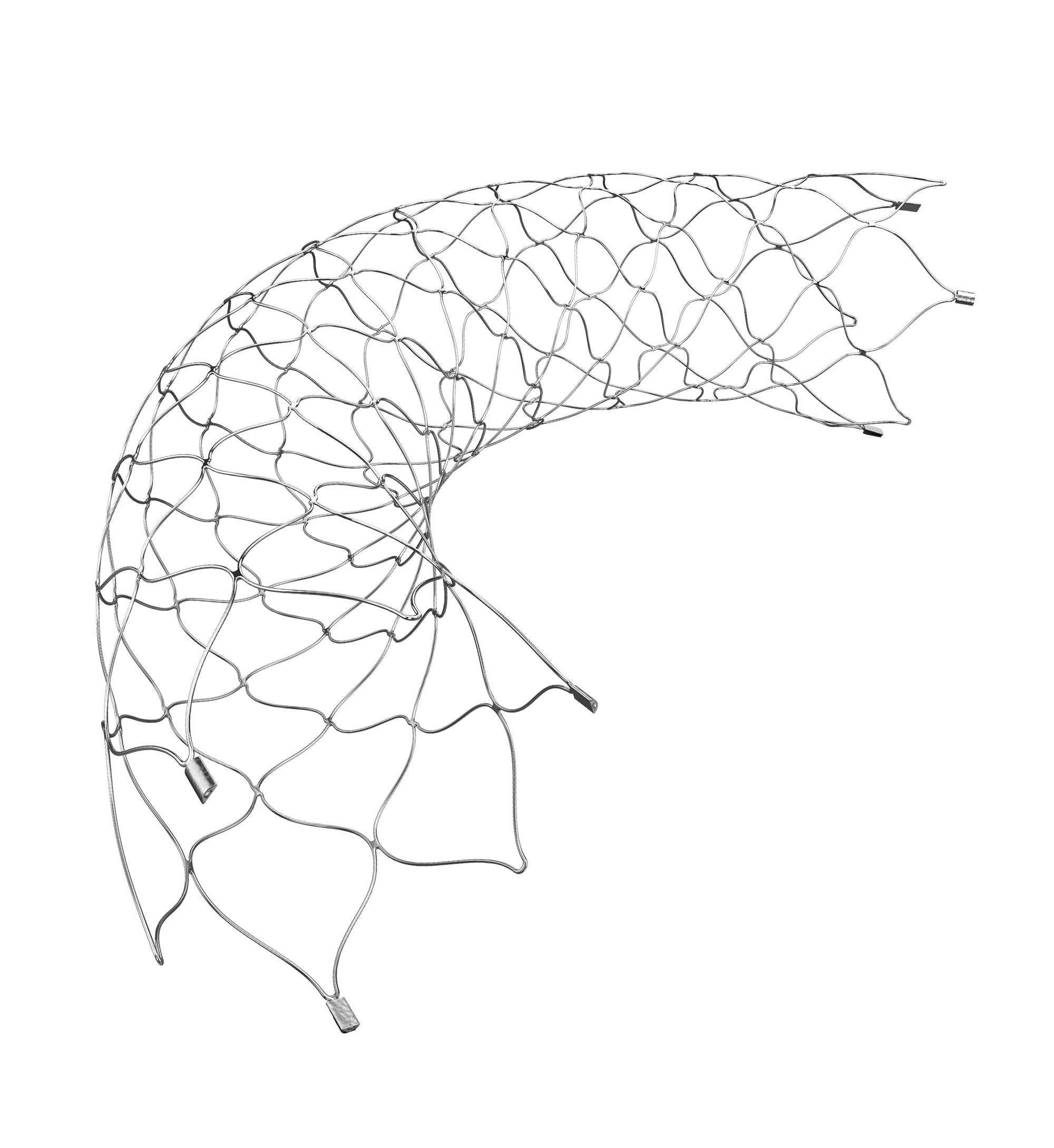
ACCLINO® flex plus
Stent
Lasercut, self-expanding nitinol stent
- Patented asymmetric closed cell design for high flexibility
- Excellent wall positioning, reliable opening behaviour
- Very good visibility due to platinum markers
- CE mark approved for vessel diameters from 1.5 - 7.0 mm
- Stents with diameters up to 5.5 mm deliverable through 0.0165’’ ID microcatheters
- Electropolished surface with low thrombogenicity*
- Repositionable up to 90 % of its length
- Also available with unique HEAL Technology
*Results from in-vitro tests
Note: Magnetic Resonance Imaging (MRI) Information
Non-clinical tests have shown that the Acandis implant is suitable for MR-examination. After implantation, patients can be safely scanned with a static magnetic field of 3 Tesla. The MR imaging quality may be affected if the implant is located in the area of interest. Optimisation of the imaging parameters is recommended. For further information on MRI compatibility please consult the instructions for use of the respective product.
Clinical Results
100%
Technical Success
92.3%
Complete Occlusion
at early follow-up
(6 months)
11%
Retreatment
Goertz, L. et al. (2019): Long-term Angiographic Results of the Low-profile Acandis Acclino Stent for Treatment of Intracranial Aneurysms. A Multicenter Study. Clinical Neuroradiology, 2019
Find publications and clinical studies on our products.
Contact
Clinical Support (clinical-support@acandis.com)
Disclaimer:
Please consult the Instructions for Use for all indications, contraindications, warnings, cautions as well as possible adverse effects. Acandis® products are to be used exclusively by trained medical professionals. Orders are taken only in regions where the product is approved.
Please contact an Acandis® representative for product availability.