
APERIO® Hybrid
Thrombectomy Device
for fast recanalisation

APERIO® Hybrid Thrombectomy Device
Highly visible nitinol stent retriever
- Excellent full length visibility
- Hybrid cell design for successfull recanalisation*
- CE mark approved for vessel diameters from 1.5 – 5.5 mm
- Compatible with 0.021’’ – 0.027’’ ID microcatheters
* Kaschner, M. et al. (2020): The new fully radiopaque Aperio Hybrid stent retriever: Efficient and safe?
An early Multicenter Experience. World Neurosurgery, 2020, 141 (September), pp. e278-e288
Clinical Results
97.9%
Very good device visibility
95.8%
mTICI 2b-3 Overall
83.4%
mTICI 2b-3 APERIO® Hybrid
(alone)
Kaschner, M. et al. (2020): The new fully radiopaque Aperio Hybrid stent retriever:
Efficient and safe? An early Multicenter Experience.
World Neurosurgery, 2020, 141 (September), pp. e278-e288.
Find publications and clinical studies on our products.
Clinical Experience
with APERIO® Hybrid Thrombectomy Devices
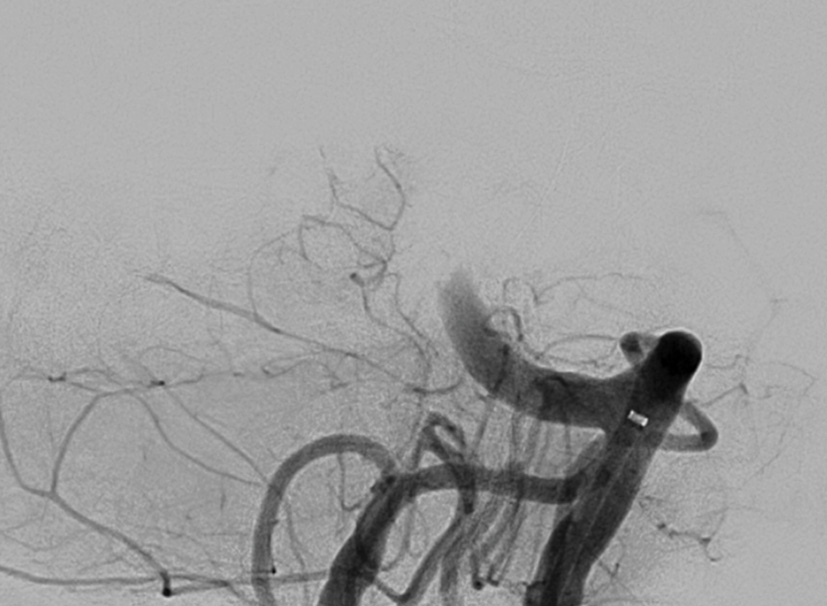
Pre treatment
Total occlusion of basilar artery
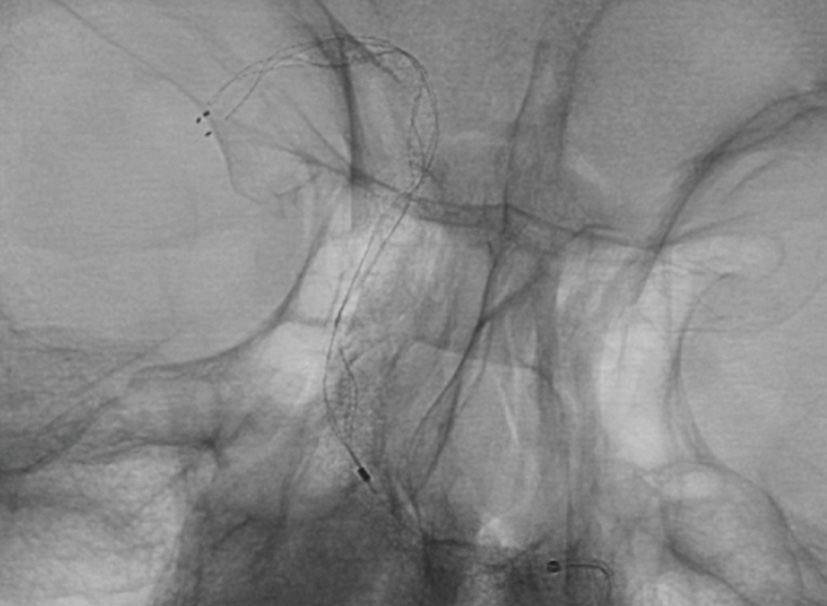
Treatment with
APERIO® Hybrid Thrombectomy Device
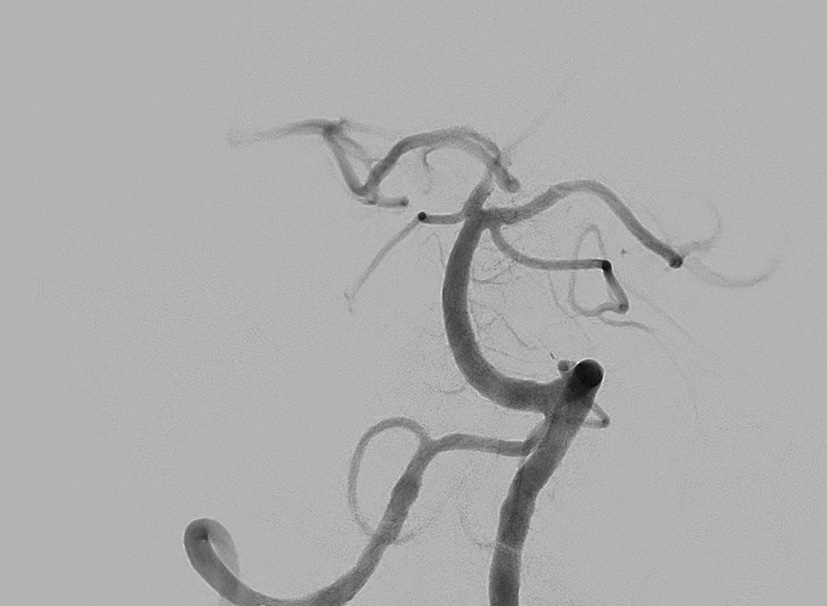
Post treatment
Final result: TICI III (first pass)
Images are courtesy of Dr. Christoph Kabbasch,
University Hospital Cologne, Germany
Contact
Clinical Support (clinical-support@acandis.com)
Disclaimer:
Please consult the Instructions for Use for all indications, contraindications, warnings, cautions as well as possible adverse effects. Acandis® products are to be used exclusively by trained medical professionals. Orders are taken only in regions where the product is approved.
Please contact an Acandis® representative for product availability.